Research

Our broad research interest lies in cellular quality control systems that detect, respond to, and resolve different forms of cellular stress — processes that are important in maintaining cellular homeostasis and overall health. However, quality control systems and stress response pathways are often compromised during aging and age-related diseases such as neurodegeneration. Our central question is whether enhancing cellular quality control systems can counteract the decline associated with aging.
In the past three years, we have built a strong research program focusing on two major directions: (1) lysosomal quality control in response to cellular stress and (2) the primordial functions of STING in cellular homeostasis. Lysosomes are known as the “longevity-promoting” organelles which degrade a wide range of macromolecules, supporting cellular growth, metabolism, and stress resilience. Stimulator of interferon genes (STING), a major signaling adaptor in innate immunity, has conserved activities in promoting cellular homeostasis, independent of its well-known functions in antiviral signaling and inflammation. Age- or disease-related stress can activate both lysosomal quality control and the cGAS (cyclic GMP-AMP synthase)-STING innate immune pathway for cellular homeostasis. However, failed lysosomal quality control and/or chronic STING activation causes pathogenic inflammation. Our short-term goal is illustrating how lysosomal integrity and activity are maintained in response to cell stress and how STING, which is also activated by diverse types of cell stress, promotes cellular homeostasis through its proton channel activity. Our long-term goal is to target lysosomal quality control pathways and STING for healthy aging.
Approach: We aim to identify the minimal set of essential components required for each biological process under investigation. To achieve our goals, we search for essential, unifying principles behind complex cellular systems through unbiased screens, and dissect the underlying mechanisms using multidisciplinary methods including molecular biology, biochemistry, cell biology, genetics, in vitro reconstitution, and multi-omics. Structural analysis and functional mutagenesis are integral parts of all our projects. Lipid signaling and membrane biology are often incorporated into all directions.
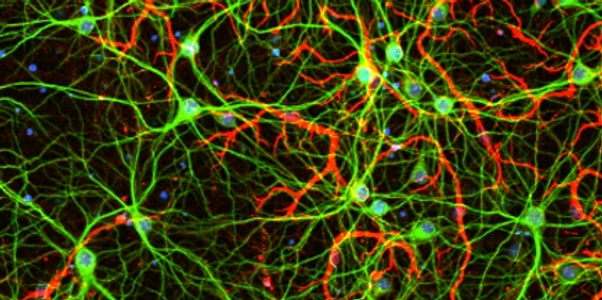
Lysosomal repair in aging and neurodegeneration
Lysosomal membrane damage is commonly triggered by diverse cellular stressors and is increasingly linked to normal aging and age-related diseases.
Our recent work uncovered the phosphoinositide- initiated membrane tethering and lipid transport (PITT) pathway as an essential mechanism for rapid lysosomal repair (Nature, 2022). Upon lysosomal membrane damage, a new phosphoinositide messenger, PI4P, is rapidly produced on lysosomes, which in turn drives the formation of extensive membrane contacts between damaged lysosomes and the endoplasmic reticulum (ER). These new inter-organelle interactions directly mediate rapid lysosomal repair, with important implications for a wide range of lysosomal-related diseases.
Tau fibril spreading is a major factor contributing to the progression of Alzheimer’s disease (AD). Lysosomal membrane damage by internalized tau fibrils is a key step in tau spreading. Our work shows that defects in PITT pathway markedly increases tau fibril spreading in cell-based assays. We are further characterizing how lysosomal quality control antagonizes tau spreading in vitro and in vivo and explore pharmacological strategies to activate this pathway for AD treatments.
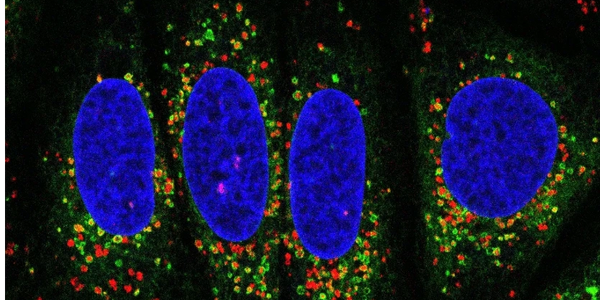
Inter-organelle communications in aging
We are interested in essential inter-organelle communications that maintain cellular homeostasis. Our recent work revealed striking ER-lysosomal interactions upon lysosomal stress. Factors recruited to these membrane contact sites include not only the rapid lysosomal repair machineries, but also additional proteins with distinct functions. We are continuing investigating how these factors might contribute to lysosomal quality control in aging and whether these inter-organelle events are triggered in aging or age-related diseases.
We are also interested in membrane contacts initiated from other organelles such as lipid droplets, peroxisomes, and mitochondria. We are developing new approaches to discover stress-induced inter-organelle tethers.
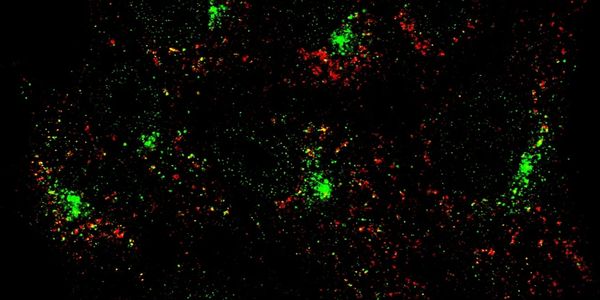
Lysosomes in senescence and age-related inflammation
Most well-known as degradative compartments, lysosomes are multifunctional centers also involved in metabolism, nutrient sensing, and immunity. Dysfunctional lysosomes are implicated in senescence and autoimmune disorders. We are using high throughput screens to search for the molecular basis of lysosomal dysfunction-induced senescence and inflammation. We are also trying to understand communications between lysosomal stress and innate immune pathways.
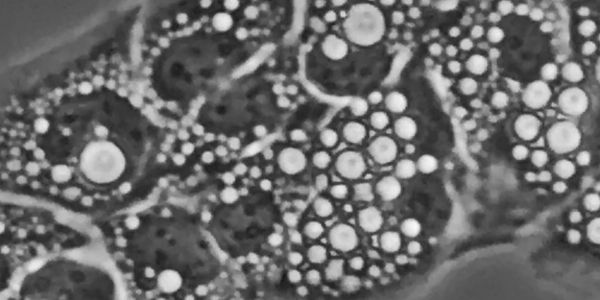
Lysosomal vacuolation in health and disease
Under various pathophysiological conditions, including lysosomal storage disorders, aging, infection, and neurodegeneration, lysosomes can form large vacuoles associated with lysosomal dysfunction. Although lysosomal vacuolation has been observed for decades and is presumed to be an adaptive stress response, its underlying mechanisms and physiological functions remain poorly understood.
Our recent work identifies PDZD8/LYVAC-mediated, directional ER-to-lysosome lipid transfer as an essential mechanism for lysosomal vacuolation (Science 2025). Diverse vacuolating stimuli converged on a common pathway involving lysosomal osmotic stress, lipid signaling, and regulation of LYVAC recruitment and lipid transfer. By linking lysosomal osmotic imbalance to vacuole formation, LYVAC underlies a robust cellular response with broad implications for lysosomal adaptation, osmoresilience, lysosomal storage pathology, and neurodegeneration.
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.