Tan Lab
Studies cellular principles of aging and age-related pathology
Studies cellular principles of aging and age-related pathology

Our broad research interest lies in cellular quality control systems that detect, respond to, and resolve different forms of cellular stress — processes that are important in maintaining cellular homeostasis and overall health. However, quality control systems and stress response pathways are often compromised during aging and age-related diseases such as neurodegeneration. Our central question is whether enhancing cellular quality control systems can counteract the decline associated with aging.
In the past three years, we have built a strong research program focusing on two major directions:
(1) lysosomal quality control in response to cellular stress and (2) the primordial functions of STING in cellular homeostasis. Lysosomes are known as the “longevity-promoting” organelles which degrade a wide range of macromolecules, supporting cellular growth, metabolism, and stress resilience. Stimulator of interferon genes (STING), a major signaling adaptor in innate immunity, has conserved activities in promoting cellular homeostasis, independent of its well-known functions in antiviral signaling and inflammation. Age- or disease-related stress can activate both lysosomal quality control and the cGAS (cyclic GMP-AMP synthase)-STING innate immune pathway for cellular homeostasis. However, failed lysosomal quality control and/or chronic STING activation causes pathogenic inflammation. Our short-term goal is illustrating how lysosomal integrity and activity are maintained in response to cell stress and how STING, which is also activated by diverse types of cell stress, promotes cellular homeostasis through its proton channel activity. Our long-term goal is to target lysosomal quality control pathways and STING for healthy aging.
Approach: We aim to identify the minimal set of essential components required for each biological process under investigation. To achieve our goals, we search for essential, unifying principles behind complex cellular systems through unbiased screens, and dissect the underlying mechanisms using multidisciplinary methods including molecular biology, biochemistry, cell biology, genetics, in vitro reconstitution, and multi-omics. Structural analysis and functional mutagenesis are integral parts of all our projects. Lipid signaling and membrane biology are often incorporated into all directions.

Lysosomal membrane damage is commonly triggered by diverse cellular stressors and is increasingly linked to normal aging and age-related diseases.
Our recent work uncovered the phosphoinositide- initiated membrane tethering and lipid transport (PITT) pathway as an essential mechanism for rapid lysosomal repair (Nature, 2022). Upon lysosomal membrane damage, a new phosphoinositide messenger, PI4P, is rapidly produced on lysosomes, which in turn drives the formation of extensive membrane contacts between damaged lysosomes and the endoplasmic reticulum (ER). These new inter-organelle interactions directly mediate rapid lysosomal repair, with important implications for a wide range of lysosomal-related diseases.
Tau fibril spreading is a major factor contributing to the progression of Alzheimer’s disease (AD). Lysosomal membrane damage by internalized tau fibrils is a key step in tau spreading. Our work shows that defects in PITT pathway markedly increases tau fibril spreading in cell-based assays. We are further characterizing how lysosomal quality control antagonizes tau spreading in vitro and in vivo and explore pharmacological strategies to activate this pathway for AD treatments.
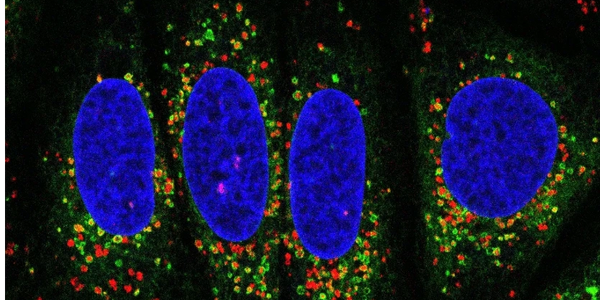
We are interested in essential inter-organelle communications that maintain cellular homeostasis. Our recent work revealed striking ER-lysosomal interactions upon lysosomal stress. Factors recruited to these membrane contact sites include not only the rapid lysosomal repair machineries but also additional proteins with distinct functions. We are continuing investigating how these factors might contribute to lysosomal quality control in aging and whether these inter-organelle events are triggered in aging or age-related diseases.
We are also interested in membrane contacts initiated from other organelles such as lipid droplets, peroxisomes, and mitochondria. We are developing new approaches to discover stress-induced inter-organelle communications.
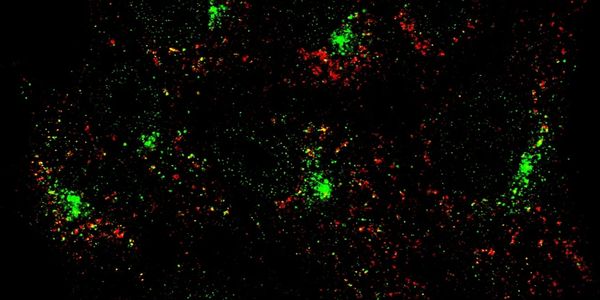
Most well-known as degradative compartments, lysosomes are multifunctional centers also involved in metabolism, nutrient sensing, and immunity. Dysfunctional lysosomes are implicated in senescence and autoimmune disorders. We are using high throughput screens to search for the molecular basis of lysosomal dysfunction-induced senescence and inflammation. We are also trying to understand communications between lysosomal stress and innate immune pathways.
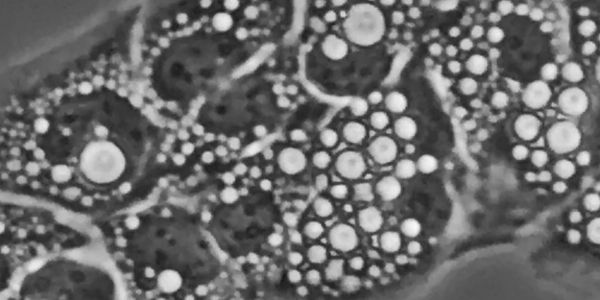
Under various pathophysiological conditions, including lysosomal storage disorders, aging, infection, and neurodegeneration, lysosomes can
form large vacuoles associated with lysosomal dysfunction. Although lysosomal vacuolation has been observed for decades and is presumed to be an adaptive stress response, its underlying mechanisms and physiological functions remain poorly understood.
Our recent work identifies PDZD8/LYVAC-mediated, directional ER-to-lysosome lipid transfer as an essential mechanism for lysosomal vacuolation (Science 2025). Diverse vacuolating stimuli converged on a common pathway involving lysosomal osmotic stress, lipid signaling, and regulation of LYVAC recruitment and lipid transfer. By linking lysosomal osmotic imbalance to vacuole formation, LYVAC underlies a robust cellular response with broad implications for lysosomal adaptation, osmoresilience, lysosomal storage pathology, and neurodegeneration.
Research articles
Yang H, Xun J, Li Y, Mondal A, Lv B, Watkins S, Shi L, Tan JX. LYVAC/PDZD8 is a lysosomal vacuolator. Science. 2025 Aug 21;389(6762):eadz0972. doi: 10.1126/science.adz0972
Highlight: A protein tunnel helps stressed lysosomes swell. Lippincott-Schwartz J.Science. 2025 Aug 21;389(6762):782-783. doi: 10.1126/science.aea5377.
Lv B, Dion WA, Yang H, Xun J, Kim DH, Zhu B, Tan JX. A TBK1-independent primordial function of STING in lysosomal biogenesis. Molecular Cell. 2024 Sep 19.
Highlight: TFEB links the cGAS-STING pathway to lysosome biogenesis
Jinrui Xun, Zhichao Zhang, Bo Lv, Defen Lu, Haoxiang Yang, Guijun Shang, Jay Xiaojun Tan. A conserved ion channel function of STING mediates noncanonical autophagy and cell death. EMBO Reports. published online. Jan 2, 2024.
Highlight: STINGing organelle surface with acid
Tan, JX, Finkel, T. A phosphoinositide signalling pathway mediates rapid lysosomal repair. Nature. (2022). https://doi.org/10.1038/s41586-022-05164-4
Highlights:
A quick fix for lysosomes. Nat Struct Mol Biol 29, 955 (2022).
Phosphoinositide signal for lysosomal membrane repair. Nat Rev Mol Cell Biol 23, 697 (2022).
PITTching in for lysosome repair. Dev Cell. 2022
The PITT pathway: Keeping lysosomes young. Clin Transl Med 12(10): e1097 (2022).
Gui X, Yang H, Li T, Tan X, Shi P, Du F, Chen ZJ. Autophagy Induction via STING Trafficking Is a Primordial Function of the cGAS Pathway. Nature. 2019;567(7747):262-266.
Tan X, Thapa N, Sun Y, Anderson RA. A kinase independent role for EGF receptor in autophagy initiation. Cell. 2015;160(1-2):145-60.
Tan X, Thapa N, Liao Y, Choi S, Anderson RA. PtdIns(4,5)P2 signaling regulates ATG14 and autophagy. PNAS. 2016;113(39):10896-901. doi: 10.1073/pnas.1523145113.
Tan X, Sun Y, Thapa N, Liao Y, Hedman AC, Anderson RA. LAPTM4B is a PtdIns(4,5)P2 effector that regulates EGFR signaling, lysosomal sorting, and degradation. EMBO J. 2015; 34(4):475-90.
Reviews/Comments/Perspectives
Tan, J.X. Harnessing lysohormesis for healthy ageing. Nature Cell Biology, 2025. March 24: 1-4.
Xun J, Tan JX. Lysosomal Repair in Health and Disease. Journal of Cellular Physiology. 2025. 240 (5), e70044.
Bo Lv, Jinrui Xun, Jay Xiaojun Tan. STING-dependent ATG8ylation. 2024. Book Title: Atg8ylation And Its Manifestations, Edited By Vojo Deretic. ISBN:978-1-0364-0892-3. Cambridge Scholars Publishing
Tan JX*, Finkel T. Lysosomes in senescence and aging. EMBO Reports. 2023:e57265. *Correspondence.
Yang H, Tan JX. Lysosomal quality control: molecular mechanisms and therapeutic implications. Trends in Cell Biology. 2023. DOI:https://doi.org/10.1016/j.tcb.2023.01.001
Tan X, Anderson RA. Keeping in touch with the ER network. Science. 2017;12;356(6338): 584-585.
Tan JX, Finkel T. Autophagy goes nuclear. Nature Cell Biology. 2020;22(10):1159-61.
Tan JX, Finkel T. Mitochondria as intracellular signaling platforms in health and disease. Journal of Cell Biology. 2020;219(5): e202002179.
Tan X, Sun L, Chen J, Chen ZJ. Detection of microbial infections through innate immune sensing of nucleic acids. Annual Review of Microbiology. 2018;72:447-78.
Tan X, Lambert PF, Rapraeger AC, Anderson RA. Stress-induced EGFR trafficking: mechanisms, functions, and therapeutic implications. Trends Cell Biol. 2016;26(5):352-66.
Complete List of Published Work: Pubmed Google Scholar
Complete List of Published Work: Pubmed Google Scholar

Undergraduate researcher

Undergraduate Researcher

Research Assistant Professor

Postdoctoral Researcher

Undergraduate Researcher

MD student; Xiangya Scholar

Undergraduate Researcher

Postdoctoral Researcher
Huge congratulations to Haoxiang and Jeff, our very first two students, on publishing their groundbreaking research in Science!

The first transcription program downstream of the primordial STING channel is published in Molecular Cell. Congratulations to Bo & coauthors!
The peer-reviewed version of the "STING channel" work is online today at EMBO Reports. Congratulations to Jeff and Bo!

We bioRxiv'ed a manuscript - A conserved ion channel function of STING in non-canonical autophagy and cell death. Congratulations to all the co-authors!
Our recent work on lysosomal repair is published online today at Nature. Congratulations!
Genetic Engineering & Biotechnology News: Longevity Depends on Prompt Repair of Lysosomal Breaches (genengnews.com)
学术经纬:今日《自然》:两位科学家首次揭示溶酶体修复的核心机制
BioArt: Nature | 溶酶体损伤修复的核心机制
We are seeking talented and passionate scientists from all stages to join our team. We support all team members to succeed in both research and career. Training and mentoring are important aspects integrated into all team members' career development plans.
If you are interested in joining us, please email jay.tan at pitt.edu a cover letter (summarizing your previous training, research interests, and career goals), CV, and contact information for three references.
We are searching for a highly motivated postdoctoral fellow to join us in studying lysosomal stress in innate immunity and age-related inflammation. Excellent training opportunities will be provided in both research and career development as well as in exploring new directions with established screening approaches in the lab. The candidate is expected to seek independent positions in academia when moving forward from this position.
We are recruiting graduate students and research technicians with 0-3 years of research experience to join us in studying lysosomal quality control in aging and Alzheimer's disease. We have established a robust research program on these topics and will provide extensive training in cell biology, molecular biology, biochemistry, advanced biochemistry, genetics, and mouse models.
Research technicians should have a bachelor's degree and are expected to apply for Ph.D. or M.D. programs when moving forward from the lab.
We have open positions for Tsinghua and Xiangya Scholars in studying cellular quality control mechanisms in aging, immunity, and disease. We have multiple active research projects on these topics and will provide top-quality training in cell biology, molecular biology, biochemistry, advanced biochemistry, genetics, and mouse models.
Multiple undergraduate research positions are available for our exciting, ongoing projects. Undergraduate researchers are expected to apply for Ph.D. or M.D. programs when moving forward to the next stage of their careers.
Assistant Professor
Aging Institute
Department of Cell Biology
University of Pittsburgh School of Medicine/UPMC
Bridgeside Point I, Suite 564
100 Technology Dr.
Pittsburgh, PA 15219
Phone: 412-624-2291
Fax: 412-383-9055
Email: Jay.Tan at Pitt.edu
100 Technology Drive, Room 457, Pittsburgh, Pennsylvania 15219, United States
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.